Clean air is considered a basic requirement for human health and well-being. However, air pollution continues to pose a major threat to health around the world. The World Health Organisation estimates that air pollution contributes to or causes 4.6 million deaths each year. The harsh reality of air pollution is that it is generally much worse than most of us know. Citizen Science initiatives are one of the possible ways to take action on this issue.
¿What does Sensora measure?
Carbon monoxide
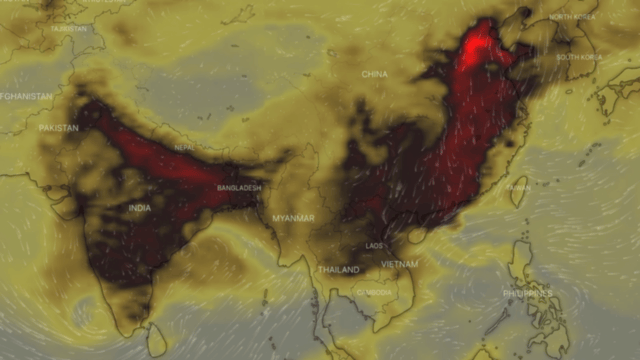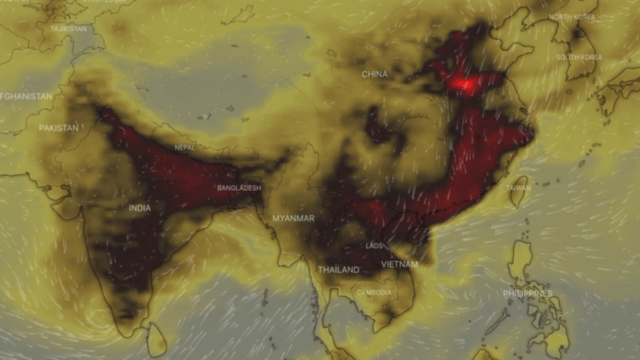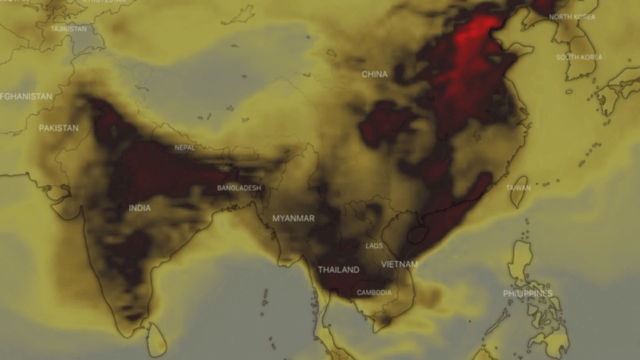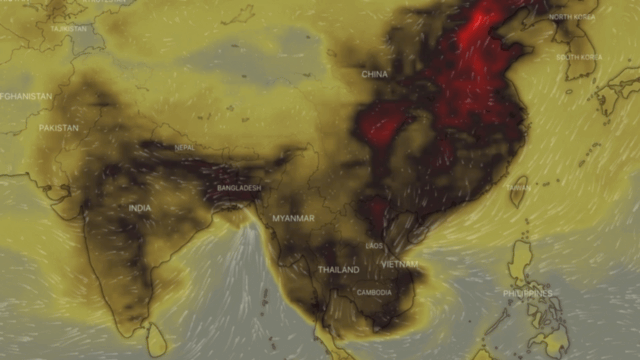
Carbon monoxide or CO, also called carbon oxide, carbon dioxide, carbon dioxide gas and carbon dioxide anhydride, is a colourless, highly toxic gas. It results from the poor combustion of gas, gasoline, paraffin, coal, oil, tobacco and wood; from vehicles with engines running; and from malfunctioning household appliances, such as cookers, cookers and paraffin heaters.
If breathed at high levels it can cause death.
Hydrogen sulphide (or Hydrogen sulphide acid)
It is a colourless, flammable gas with a slightly sweet taste and an odour of decomposing organic matter – rotten eggs.
It is found naturally in petroleum, natural gas, in gases emitted in processes associated with volcanic phenomena and in hot springs.
It is generated by the decomposition of existing organic matter under anaerobic conditions (a process in which microorganisms break down biodegradable material in the absence of oxygen).
May exist in sewage systems, animal processing plants, landfills, sludge treatment plants, oil or natural gas drilling sites, ponds and cesspools.
Exposure to low concentrations may cause irritation of the eyes, nose, or throat; brief exposures to high concentrations may cause unconsciousness. Permanent or long-term effects may also occur, such as headaches, lapses in concentration, poor memory and impaired motor functions.
Nitrogen dioxide
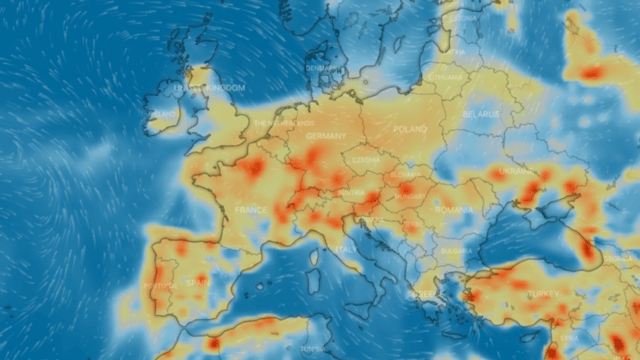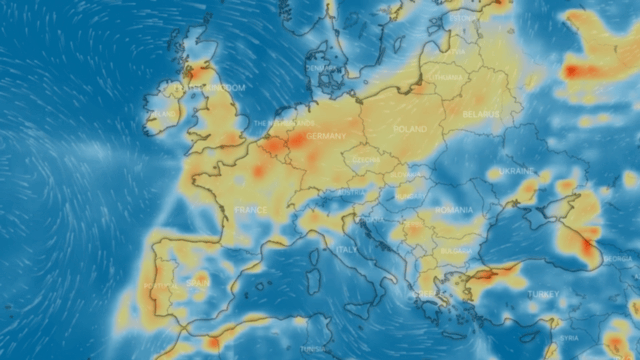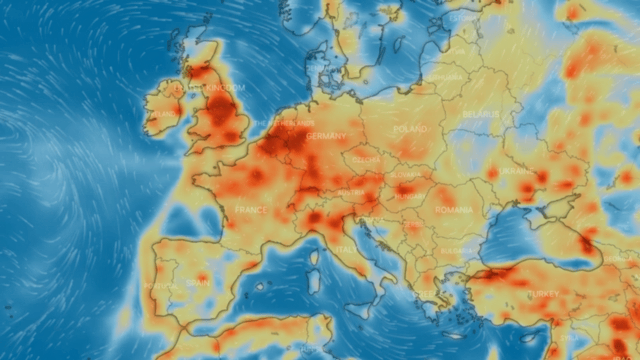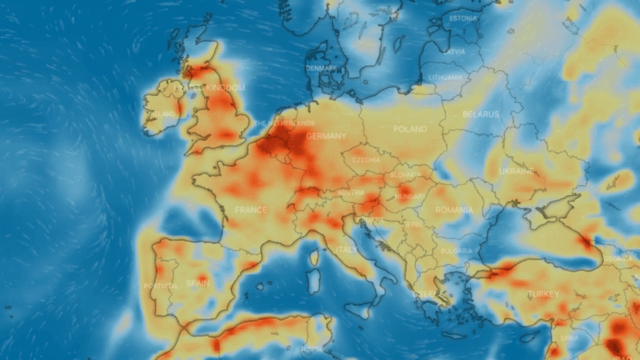
Nitrogen dioxide, or nitrogen oxide, is a yellowish-brown gaseous chemical compound formed by the combination of one atom of nitrogen and two atoms of oxygen. It is a toxic and irritating gas that is a precursor to the formation of nitrate particles. These lead to acid production and elevated levels of PM-2.5 in the environment.
It is formed as a by-product of high-temperature combustion processes, such as in motor vehicles and power plants. It is therefore a frequent pollutant in urban areas. As an air pollutant it is regulated by legal regulations in many countries.
It mainly affects the respiratory system.
High levels can irritate the lungs and decrease lung function as well as lower resistance to respiratory infections. It increases mucus in the upper respiratory tract, which can increase respiratory infections and exacerbate symptoms in patients with chronic respiratory diseases, asthmatics and allergy sufferers. Bronchitis in the elderly and immunocompromised and bronchiolitis in children.
Ozone
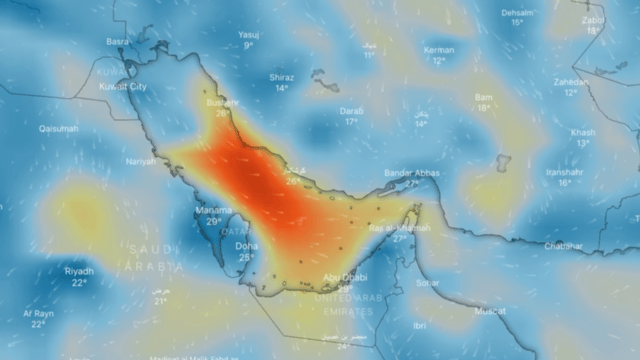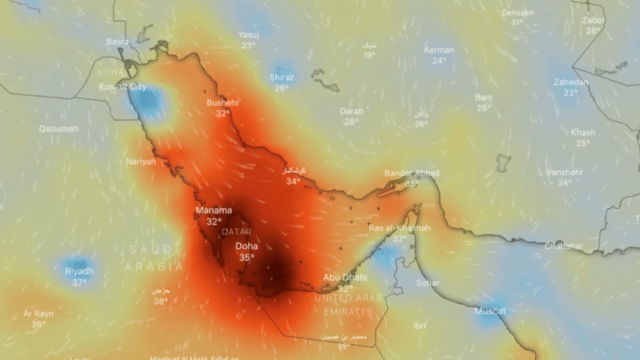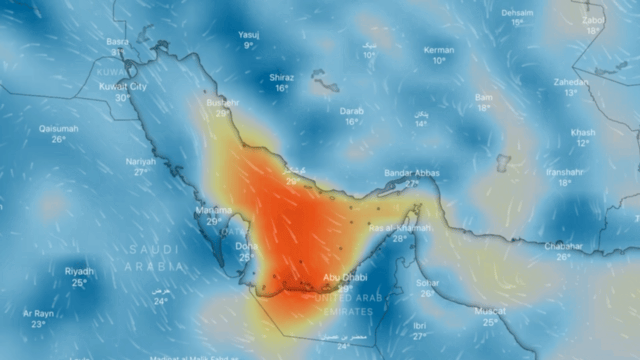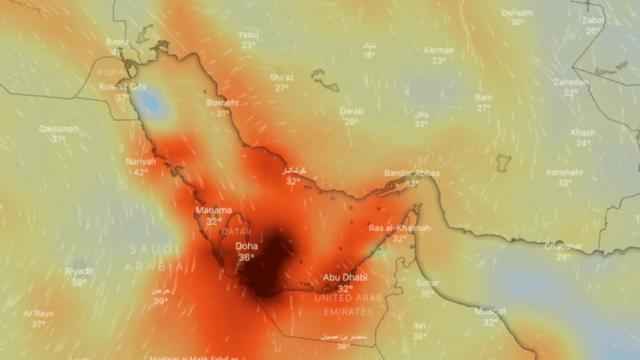
Ozone (O3) is an unstable gas, which consists of 3 oxygen atoms (O), formed by the dissociation of the two atoms that make up oxygen gas.
At ambient temperature and pressure, ozone is a gas that gives off a strong odour (similar to that of seafood in an advanced state of decomposition) and is generally colourless, but in large concentrations it can turn slightly bluish.
If breathed in large quantities it can cause coughing, throat irritation, worsening of conditions such as asthma, bronchitis and emphysema, and even permanent lung damage. At very low concentrations, it may be harmful to the upper respiratory tract and lungs, even for short-term exposure; at higher concentrations reduced kidney function, extreme fatigue, dizziness, inability to sleep or cyanosis may occur.
A temperatura y presión ambientales, el ozono es un gas que desprende olores fuertes (similar al de los mariscos en estado de descomposición avanzado) y generalmente sin coloración, pero en grandes concentraciones puede volverse ligeramente azulado.
Si se respira en grandes cantidades puede provocar tos, irritación en la garganta, empeoramiento de afecciones como asma, bronquitis y enfisema y hasta daños pulmonares permanentes. En concentraciones muy bajas, puede ser nocivo para el tracto respiratorio superior y los pulmones, aun tratándose de una exposición de corta duración; a concentraciones más altas puede aparecer función renal reducida, fatiga extrema, mareo, inhabilidad para dormir o cianosis.
Particulate matter PM 10 and PM 2.5
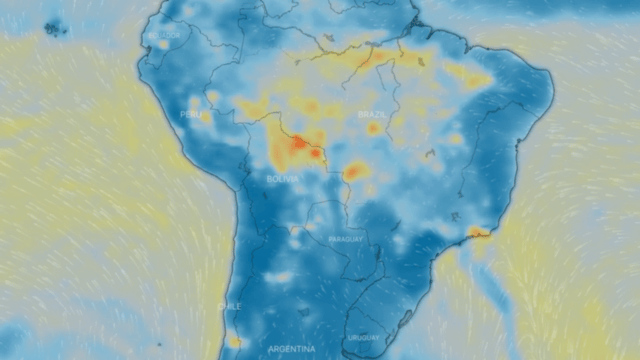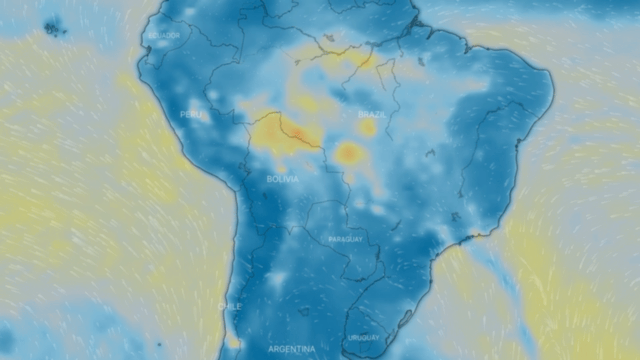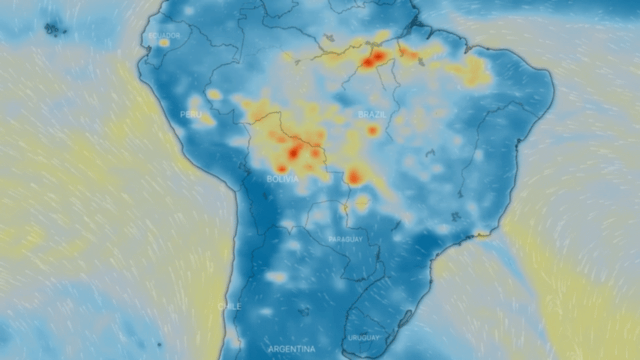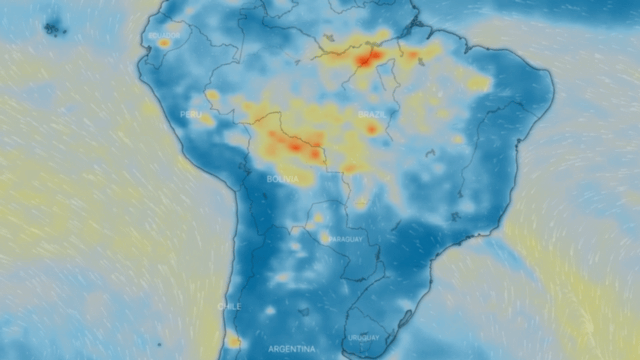
These are a series of tiny solid bodies or liquid droplets dispersed in the atmosphere generated by anthropogenic activity (human activity, such as burning coal to produce electricity) or natural activity (volcanic activity). Their composition is very varied and we can find, among their main components, sulphates, nitrates, ammonia, sodium chloride, coal, mineral dust, metallic ashes and water. These particles also produce chemical reactions in the air.
They are generated by the incomplete combustion of carbon-based fuels, such as coal or charcoal, oil, gasoline and diesel, giving rise to soot; fine particulates also contain heavy metals.
One source of both fine and coarse carbonaceous atmospheric particulate matter is vehicle exhaust, especially diesel-powered vehicles. Another type of important fine particulate matter suspended in the atmosphere consists predominantly of inorganic sulphur and nitrogen compounds.
PM10 or inhalable particles
PM 2.5 or respirable particles.
PM10 is behind numerous respiratory diseases, cardiovascular problems and lung cancer.
PM 2.5 irritates the airways, causing coughing or shortness of breath; it tends to affect mostly people with heart or lung disease, children and older adults
